Over-the-Counter (OTC) Hearing Aids in the USA – Access and Key Features
Over-the-counter (OTC) hearing aids in the USA are devices that can be obtained directly without a medical prescription. These aids are designed for adults with perceived mild to moderate hearing difficulties. They include essential components such as microphones, amplifiers, and batteries, and their use should follow product instructions. All OTC hearing aids are regulated under FDA guidelines to ensure compliance with safety and quality standards.
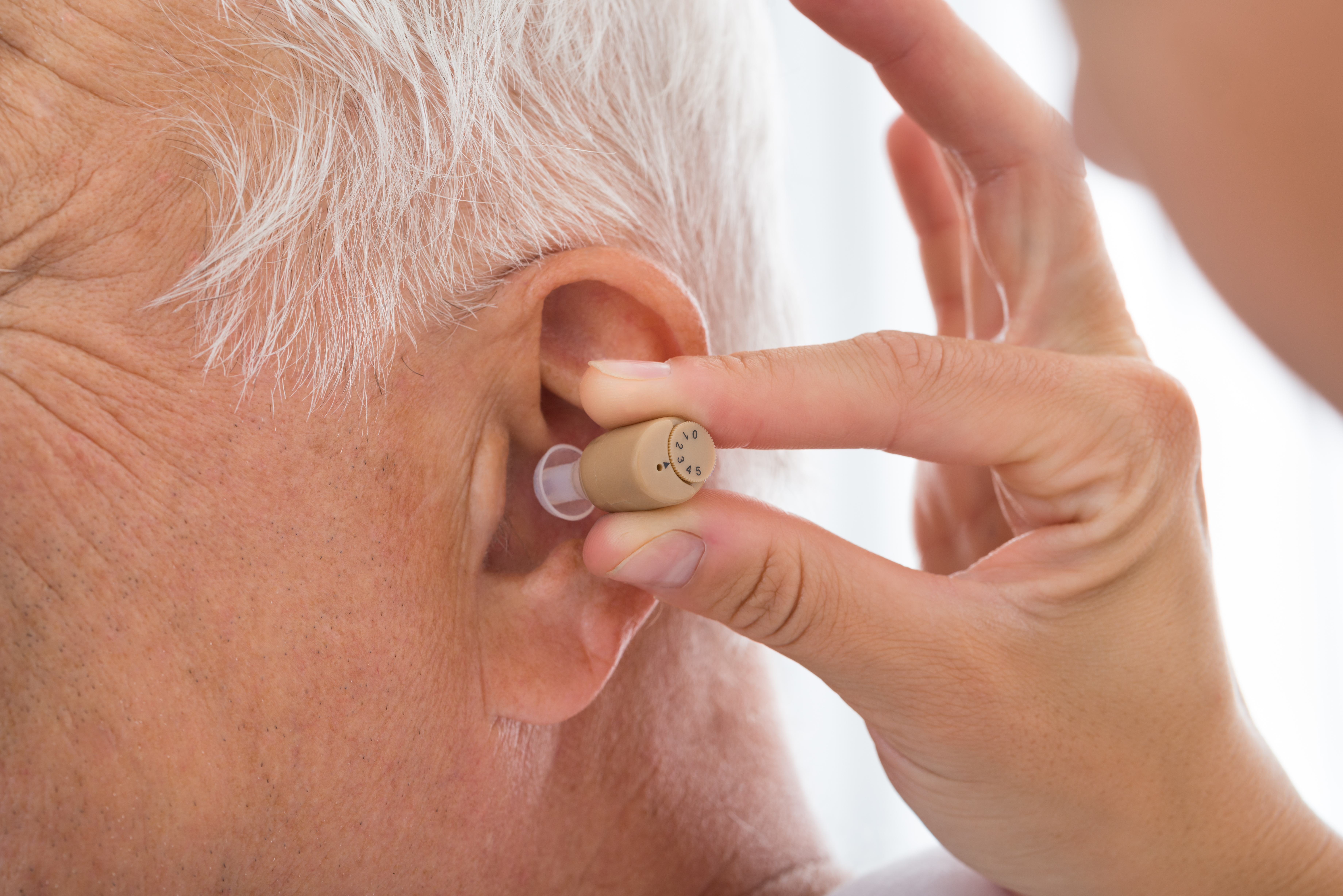
Definition and Purpose of OTC Hearing Aids in the USA
OTC hearing aids are medical devices designed to amplify sounds for adults with perceived mild to moderate hearing loss. Unlike traditional prescription hearing aids that require professional fitting and adjustment, OTC devices can be purchased directly by consumers at pharmacies, retail stores, or online without requiring a medical exam, prescription, or fitting by an audiologist. The primary purpose of establishing this new category was to improve accessibility and affordability of hearing technology for millions of Americans. These devices are specifically intended to address the significant gap in hearing aid adoption, where only about 20% of individuals who could benefit from hearing aids actually use them, largely due to barriers including cost, access to specialists, and stigma.
Design and Main Components of Hearing Aid Devices
OTC hearing aids, like their prescription counterparts, consist of several key components working together to amplify sound. The basic components include:
-
Microphone: Captures environmental sounds and converts them to electrical signals
-
Amplifier: Increases the strength of these signals based on the user’s hearing needs
-
Speaker (receiver): Converts amplified signals back into acoustic energy delivered to the ear
-
Battery: Powers the device, which may be rechargeable or disposable
-
Housing: Contains the electronic components in a durable, often water-resistant casing
OTC devices come in various form factors, including behind-the-ear (BTE), in-the-ear (ITE), and in-the-canal (ITC) designs. Many modern OTC hearing aids also incorporate digital signal processing technology that can reduce background noise, enhance speech clarity, and adjust to different listening environments. Some feature smartphone connectivity for user adjustments and personalization through dedicated apps, allowing users to create custom hearing profiles for different situations.
Intended Users and Usage Conditions for OTC Products
OTC hearing aids are specifically authorized for adults (18 years and older) with perceived mild to moderate hearing loss. They are not intended for children or for people with severe hearing loss, which requires professional assessment and more sophisticated devices. Appropriate candidates for OTC hearing aids typically experience symptoms such as:
-
Difficulty understanding conversations in noisy environments
-
Frequently asking others to repeat themselves
-
Turning up the volume on TVs or phones beyond what others find comfortable
-
Trouble hearing on the telephone
-
Finding that others seem to mumble
It’s important to note that certain conditions require professional evaluation rather than self-treatment with OTC devices. These include sudden hearing loss, significant difference in hearing between ears, tinnitus (ringing) in only one ear, pain or discomfort in the ears, or hearing loss that fluctuates significantly. The FDA recommends consumers consult with healthcare professionals if they’re unsure about the severity of their hearing loss or if they experience any of these warning signs.
FDA Regulatory Framework for OTC Hearing Aids
The FDA established the OTC hearing aid category through the Over-the-Counter Hearing Aid Act, which was part of the FDA Reauthorization Act of 2017. After a significant period of development and public comment, the final rule was implemented in October 2022. This framework:
-
Defines OTC hearing aids as a new category of regulated medical devices
-
Establishes specific performance and design requirements for safety and effectiveness
-
Provides labeling requirements to ensure consumers understand device limitations
-
Removes the requirement for medical evaluation or prescription
-
Preempts state-level regulations that would restrict OTC hearing aid sales
The FDA regulatory framework balances consumer access with safety considerations. Manufacturers must submit documentation demonstrating compliance with various technical standards before marketing their products as OTC hearing aids. The devices must also have clear labeling that includes warnings, advisories on when to consult a healthcare provider, and information about device controls and functions. This regulatory approach aims to maintain quality standards while increasing market competition and innovation.
Compliance with Safety and Product Standards
OTC hearing aids must meet specific safety and performance standards established by the FDA. These include:
-
Output sound pressure level (SPL) limitations to prevent further hearing damage
-
Electroacoustic performance requirements ensuring appropriate amplification
-
Design requirements for user controls and device construction
-
Electromagnetic compatibility standards to prevent interference with other devices
-
Requirements for clear, consumer-friendly labeling and instructions
Manufacturers must also establish quality management systems and submit documentation to the FDA demonstrating compliance with these standards. The FDA has streamlined the process for manufacturers by exempting OTC hearing aids from certain requirements that apply to prescription devices, while still maintaining core safety protections. OTC devices must include user-adjustable volume controls and a means for the user to customize the device’s frequency response to accommodate their specific hearing needs. Additionally, manufacturers must provide information about device care, maintenance, troubleshooting, and expected battery life.
Pricing and Availability of OTC Hearing Aids
The introduction of OTC hearing aids has significantly impacted the hearing aid market by creating more affordable options compared to traditional prescription devices. While prescription hearing aids often cost between $2,000 and $5,000 per ear, OTC alternatives generally range from $200 to $1,000 per pair, representing substantial savings for consumers.
| Brand | Model | Features | Price Range |
|---|---|---|---|
| Lexie | B2 Powered by Bose | Self-fitting, smartphone app, rechargeable | $899/pair |
| Sony | CRE-C10 | Self-fitting, smartphone app, disposable batteries | $999/pair |
| Eargo | Neo HiFi | Nearly invisible design, rechargeable, app controls | $1,950/pair |
| Audien | Atom Pro | Simple controls, rechargeable, no app required | $249/pair |
| Jabra | Enhance Select 50 | Customizable settings, water-resistant, rechargeable | $799/pair |
Prices, rates, or cost estimates mentioned in this article are based on the latest available information but may change over time. Independent research is advised before making financial decisions.
Conclusion
OTC hearing aids represent a significant advancement in hearing healthcare accessibility in the United States. By removing barriers to access and reducing costs, these devices have the potential to help millions of Americans with mild to moderate hearing loss who previously went untreated. While OTC options provide greater consumer choice and control, they also place more responsibility on users to select appropriate devices. The FDA’s regulatory framework balances this increased access with necessary safeguards to ensure device safety and effectiveness. As the market continues to evolve, consumers will likely benefit from ongoing innovation and competition in this growing segment of hearing healthcare products.
This article is for informational purposes only and should not be considered medical advice. Please consult a qualified healthcare professional for personalized guidance and treatment.